Our Product.
- Overview
- Applications
- Spesification
Overview
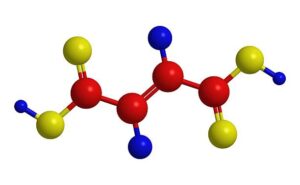
Fumaric acid is the chemical compound with the formula HO2CCH=CHCO2H. This colorless crystalline compound is one of two isomeric unsaturated dicarboxylic acids, the other being malefic acid wherein the carboxylic acid groups are cis. It has a fruit-like taste. Fumaric acid is found in fumitory (Fumaria officinalis), bolete mushrooms (specifically Boletus fomentarius var. pseudo-igniarius), lichen, and moss related to malic acid, the ionized form of fumaric acid, fumarate, is used by cells (in the TCA cycle) to produce energy from food. Human skin naturally produces fumaric acid when exposed to sunlight.
Fumaric acid is inexpensive, so it is typically purchased rather than prepared. It was first prepared from bromosuccinic acid. A traditional synthesis involves oxidation of furfural(from the processing of maize) using sodium chlorate in the presence of a vanadium-based catalyst. The chemical properties of fumaric acid can be anticipated from its component functional groups. This weak acid forms a diester, it undergoes additions across the double bond, and it is an excellent dienophile.
Biochemical aspects
Fumaric acid is a normal constituent of tissues as an intermediate in the tricarboxylic acid cycle. Distribution of fumaricacid in rat tissue has been studied by partition chromatography and it was found that blood contained 3 mg/l, brain tissue 150 mg/kg, kidney tissue 95 mg/kg, liver 78 mg/kg and muscle 23 mg/kg. Contact us today to find the best solutions for your requirements.

Applications
Fumaric acid is used as a flavoring, because it is the sourest tasting of the organic acids. Three parts of fumaric acid are as sour as five parts of citric acid. It is also used as an anti-oxidant, as a mordant (a substance that helps dyes stick to fabric), and as a buffering agent (helps maintain a particular acidity or alkalinity).
Fumaric acid is used to lower the pH (make something more acid, and thus taste more sour). This helps certain anti-microbial agents such as sodium benzoate and calcium propionate work better.Fumaric acid itself kills bacteria also. Fumaric acid breaks the sulfur-to-sulfur bonds in the elastic protein gluten in bread doughs. This makes the doughs more machineable. It also is uses in rye and sourdough breads to make them more sour.
Fumaric acid is used in combination with sodium bicarbonate to create delayed action leavening agents (something that produces carbin dioxide gas to make breads rise). Since it only dissolves in warm water, the leavening action is delayed until the bread starts to bake. Because fumaric acid is not very soluble in water, it can replace hygroscopic acids in dry mixes, and thus help keep them from caking in humid conditions.


Spesifications
| PRODUCT IDENTIFICATION | |
| CAS NO. | : 110-17-8 |
| EINECS NO. | : 203-743-0 |
| FORMULA | : HOOCCH=CHCOOH |
| MOL WT. | : 116.07 |
| H.S. CODE | : 2917.19 |
| TOXICITY | : Oral rat LD50: 9300 mg/kg |
| SYNONYMS | : 2-Butenedioic acid; 1,2-Ethylenedicarboxylic Acid;Allomaleic Acid; trans-Butanedioic Acid; (E)-2-Butenedioic acid; trans- 1,2-Ethylenedicarboxylic acid; Allomaleic acid; Boletic acid; |
| PHYSICAL AND CHEMICAL PROPERTIES | |
| PHYSICAL STATE | : Clear Crystalline Powder |
| MELTING POINT | : 287 – 302 C (Sublime) |
| SPECIFIC GRAVITY | : 1.635 |
| SOLUBILITY IN WATER | : Slightly soluble (0.63 g/100ml at 20 C) |
| AUTOIGNITION | : 740 C |
| NFPA RATINGS | : Health: 2; Flammability: 1; Reactivity: 0 |
| STABILITY | : Stable under ordinary conditions. |
| SALES SPECIFICATION | |
| TECH GRADE | |
| APPEARANCE | : white crystalline powder |
| PURITY | : 99.5% min |
| ASH | : 0.05% max |
| MELTING POINT | : 287 – 302 C |
| FOOD GRADE | |
| APPEARANCE | : white crystalline powder |
| PURITY | : 99.5% min |
| ASH | : 0.05% max |
| MELTING POINT | : 287 – 302 C |
| COLOR | : Clear trasparent (5wt% in Sodium Hydroxide) |
| SUIFATE | : 100ppm max |
| HEAVY METALS | : 10ppm max |
| ARSENIC ACID | : 3ppm max |
| MALIC ACID | : 0.1% max |
| WATER | : 0.5% max |